Barium sulfate scale inhibition in deepwater cold environments
One of the flow assurance challenges facing the deepwater oil and gas field development and production is mineral scale control. In a deepwater production system, barium sulfate scale deposition may arise from two causes, namely:
- Commingling of injected seawater with connate formation water
- Temperature cooling from bottomhole to wellhead and then in a long subsea flowline.
The first phenomenon is well understood in the offshore oil industry. However, there is little knowledge of barium sulfate scaling and inhibition mechanisms in a deepwater subsea system, where the fluid temperature can be cooled to a few degrees Celsius. This is hampered by the lack of barium sulfate solubility data at these temperatures.
To fill the gap in barium sulfate solubility data relevant to deepwater subsea temperatures, barium sulfate solubilities were measured in BaSO4-NaCl-H2O system at 5°C. Sodium chloride concentrations ranged from 0 to 5 molal.
Previously investigated barium sulfate scale formation and inhibition as a function of temperature, ranging from 95°C to 23°C was found that, for a given brine, barium sulfate scaling tendency increases significantly as temperature decreases, and scale inhibitor effectiveness (expressed as %BaSO4 Inhibition) reduces correspondingly.
The apparent loss of inhibitor efficacy at colder temperatures is mainly a result of increased barium sulfate scaling tendency (supersaturation) at lower temperatures. In contrast, by maintaining the same barium sulfate supersaturation and equilibrium precipitation (by altering sulfate ion concentration in the test brines at different test temperatures), inhibitor effectiveness moderately improves as temperature lowers.
This new finding extends the previous work on barium sulfate precipitation and inhibition to the deepwater subsea temperature (5°C). This current investigation includes kinetics of barium sulfate precipitation and its inhibition both under static conditions (jar tests) and at dynamic flow conditions (by tube-blocking tests).
Discussion
Static and dynamic precipitation experiments show barium sulfate precipitation rate in a given brine decreases as temperature cools, even though barium sulfate supersaturation increases. The precipitation rate reduces more rapidly with temperature decrease if supersaturation remains constant between the temperatures.
In static inhibition tests using the same brine at different temperatures, percentage inhibition of barium sulfate showed more precipitation from 95°C to 5°C, more with diethylenetiamine penta methylene phosphonic acid (DETPMP) than with sulfonated polymaleic acid co-polymer (S-PMA).
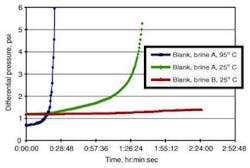
However, in the dynamic flow experiments, barium sulfate scale deposition rate in the presence of DETPMP decreased considerably with temperature reduction, even though barium sulfate supersaturation (thermodynamic scaling tendency) increased. Also in the dynamic flow experiments, barium sulfate deposition rate in a given brine with S-PMA was almost unchanged between the two temperatures (95°C to 25°C), but it was slower at the lower temperature when barium sulfate supersaturation and amount of equilibrium precipitation were kept constant.
The following postulation may explain the above results. In the static tests, the thermodynamics (barium sulfate supersaturation) is more dominant a factor than the kinetics. On the other hand, under flowing conditions in the tube-blocking tests, the kinetics are more significant a factor in scale deposition than the thermodynamics (barium sulfate supersaturation). The interplay of these two factors affects both scale formation and its inhibition by chemicals.
Static, dynamic results
Both the static and dynamic experiments agree that, at constant barium sulfate supersaturation and the amount of equilibrium precipitation, percentage inhibition of barium sulfate by either DETPMP or S-PMA improves at colder temperatures. This is likely because of the slower kinetics of barium sulfate precipitation at lower temperatures. This may not be the only factor.
As seen in the tube-blocking experiments, DETPMP performed much better at 25°C than at 95°C, while temperature did not exert such a dramatic effect on S-PMA inhibition of barium sulfate (although in static inhibition performance both inhibitors had a more similar trend with respect to temperature change). This difference may imply that:
- The two inhibitors exhibit different scale inhibition mechanisms (S-PMA is known to be more a nucleation inhibitor and DETPMP more a crystal growth retarder)
- Temperature has different effects on scale nucleation and crystal growth.
To confirm such speculation, more inhibitor chemistries need to be included in further investigations. Three main factors and their varying dependence on temperature are likely causes to the varying results discussed. These factors are barium sulfate scaling thermodynamics (supersaturation and amount of precipitation), precipitation kinetics, and inhibitor function (likely dependent on inhibitor chemistry).
Recommendations
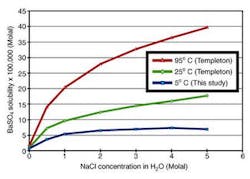
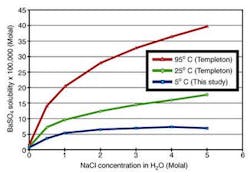
Barium sulfate solubilities in BaSO4-NaCl-H2O system at 5°C were determined as a function of sodium chloride concentration (0-5 molal). The solubility increases with ionic strength up to 4 molal NaCl then shows a moderate decrease to 5 molal NaCl. At 5°C, barium sulfate solubility is lower than that at 25°C by a factor of between 1.38 (in pure water) and 2.25 (in 5 molal NaCl solution). It is lower than that at 95°C by between 2.15 (in pure water) and 5.05 (in 5 molal NaCl). This data fills a gap in barium solubility at deepwater subsea temperatures.
The much lower barium solubility at 5°C suggests that produced water may become oversaturated with barium sulfate as temperature cools in a long subsea flowline.
Static inhibition experiments show that in a given brine, a lower percentage inhibition of barium sulfate scale is achieved at 5°C than at 95°C by the same amount of either DETPMP or S-PMA. This reduction in scale inhibition is more noticeable with DETPMP than with S-PMA.
In a flowing system (tube-blocking tests) with the same brine, DETPMP resulted in more retardation in barium sulfate scale deposition at a lower temperature than at a higher temperature, contrary to the static inhibition results. S-PMA in the brine caused little difference in deposition between the two temperatures.
When barium sulfate supersaturation and equilibrium precipitation (the thermodynamic scaling tendency) are kept unchanged, both static tests and dynamic tests show that more barium sulfate scale inhibition is attained by either inhibitor at a lower temperature than at a higher temperature. It is believed that the varying results are themselves a result of the combination of three main factors:
- Barium sulfate scaling thermodynamics (supersaturation and precipitation)
- Precipitation kinetics and inhibitor function (likely based on inhibitor chemistry)
- Individual responses to temperature change.
Because of the varying responses of those factors to temperature change, to select appropriate inhibitors for deepwater application in a cold environment, it is thus necessary to conduct scale formation and inhibition experiments using both test methods (static and dynamic) and evaluate individual inhibitors. Generalization based on one test method and/or one chemical may lead to misleading result and treatment recommendation.
Acknowledgment
Baker Petrolite granted permission to publish the data in this paper. Thomas Lopez conducted ICP analysis of barium in the experimental samples cited.
References
Alary, V., et al: "Subsea Water Separation and Injection: A Solution for Hydrates," paper OTC 12017, 2000 Offshore Technology Conference, Houston, TX, 1-4 May 2000.
Hudson, J., et al: "Flow Assurance for Subsea Wells," paper OTC 11968, 2000 Offshore Technology Conference, Houston, TX, 1-4 May 2000.
Stephens, P., et al: "Terra Nova - The Flow Assurance Challenge," paper OTC 11915, 2000 Offshore Technology Conference, Houston, TX, 1-4 May 2000.
Templeton, C.: "Solubility of Barium Sulfate in Sodium Chloride Solutions from 25°C to 95°C," J. Chem. & Eng. Data, 5(4), 514-16, 1960.
Tomson, M.: ScaleSoft Pitzer, ver2.0, Rice University, Houston, TX, 1999.
Yuan, M.: "Effect of Temperature on Barium Sulfate Scale Inhibition on Diethylenetiamine Penta (Methylene Phosphonic Acid)," 218th American Chemical Society National Meeting, New Orleans, LA, 22-26 August 1999.
Yuan, M., et al: "Investigation and Improvement of BaSO4 Scale Inhibition Tests," paper SPE 37304, 1997 SPE International Symposium on Oilfield Chemistry, Houston, TX, 18-21 February 1997.
Editor's Note: This is an updated and edited version of SPE 68311 paper, prepared for presentation at the SPE's 3rd International Symposium on Oilfield Scale, Aberdeen, UK, January 30-31, 2001.